본문
Introduction purpose
ㅇ With the current management method that manually manages production history, it is difficult and cumbersome to register defects, and it is difficult to immediately respond to quality problems due to the inability to manage production history. In addition, due to the manual recording of performance data, it is difficult to manage the data due to the production process.
ㅇ It is difficult to respond to changes in the work environment, and the current management method of manually issuing work instructions to the site and distributing paper-printed drawings to workers. It takes time to check whether
ㅇ It is difficult to systematically monitor the situation at the production site, and it is difficult to control the quality of the measured values.
ㅇ Due to the lack of coding for each product and process, it is difficult to track the lot when a defect occurs, so it takes a lot of time to analyze the cause of the defect and set up measures to prevent recurrence.
ㅇ Due to the nature of the medical device manufacturer, it is necessary to present evidence that it satisfies various domestic and international legal regulations, but due to the absence of a management system, the person in charge collects and prepares all the supporting data manually, resulting in many problems due to reduced work efficiency and low data reliability.
qualitative performance
ㅇ Minimize loss of production time that may occur due to equipment failure by real-time monitoring of the production status and establishing an environment that can respond immediately in case of failure
ㅇ In an environment where the existing performance aggregation was manually managed, performance information is aggregated based on performance data obtained through KIOSK and PDA to secure high-reliability data and result values, and to reduce the time for performance input.
ㅇ Accurate and rapid collection and analysis of production data becomes possible, and decision-making is accelerated by inquiry/output and monitoring of various reports.
ㅇ Improvement of production and shipment performance management
- Accurate and transparent performance management is possible by providing production performance management and shipment performance management through the barcode system
ㅇ It is expected that the real-time and accurate information sharing system will be established, which will increase productivity and increase corporate profits.
ㅇ Fast and consistent sharing of on-site data and expedited decision-making
ㅇ It is possible to monitor the flow and management activities within the production site such as production, inventory, defects, and materials in real time to ensure transparency. Better management is possible based on the acquired data
ㅇ Inquire about finished product production tracking information
- In order to check the tracking information of manufactured products, quickly inquire through system inquiry, not by phone or on-site inspection
ㅇ As production data can be accurately and quickly collected and analyzed, it is possible to prevent recurrence by identifying the cause of the problem.
ㅇ Fast and consistent sharing of on-site data and expedited decision-making
ㅇ Since the data obtained from the system is stored in a DB, it becomes a data asset that can be used as a basis for future business expansion and material purchase.
ㅇ Provide company-wide manufacturing status information to secure global medical device regulatory responsiveness through the introduction of global MES in the medical device industry, respond to design-manufacturing-quality linkages, and secure visibility between production/materials/quality/purchase infrastructure manufacturing execution operation and manufacturing/inventory Securing efficient management of Q (Quality)/C (Cost)/D (Schedule) from a linked perspective
ㅇ Securing CSV (Computer System Validation) and responding
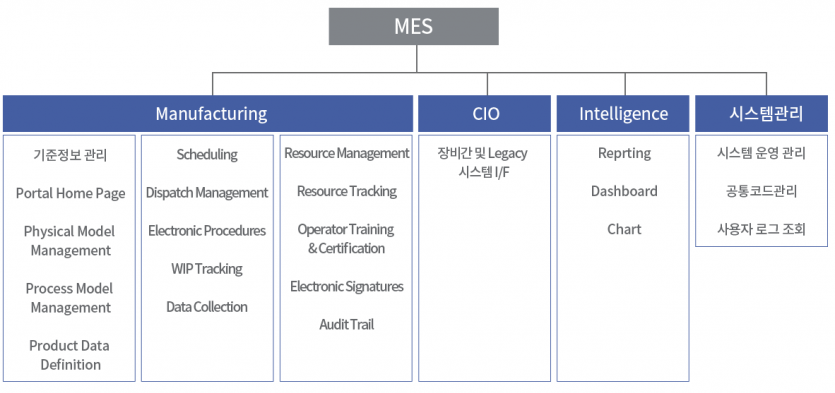
Implementation Application System






 KOR
KOR