본문
Introduction purpose
ㅇ It is difficult to manage production status, defect status, and performance aggregation because the system is not introduced to the work performed at the entire production site, such as production plan establishment, work order, and production performance aggregation.
ㅇ It is difficult to manage performance information that occurs at the production site from work orders to quality control, and it is difficult to understand the current status of production progress and status.
ㅇ It is difficult to derive the business direction due to the absence of a method for predicting and verifying the results of materials and work processes.
ㅇ Because the standard information system and BOP standard are not defined, it is difficult to cope with unexpected situations such as absence of a person in charge and an emergency order, and a work method that is dependent on the worker
ㅇ It is difficult and cumbersome to manually manage production history, so it is difficult and cumbersome to register defects, and it is difficult to immediately respond to quality problems due to the inability to manage production history.
ㅇ It is difficult to systematically monitor the situation at the production site, and it is difficult to control the quality of the measured values.
ㅇ Due to the lack of coding for each product and process, it is difficult to track the lot when a defect occurs, so it takes a lot of time to analyze the cause of the defect and set up measures to prevent recurrence.
ㅇ Data of all processes is recorded on Excel or worker A4 paper and reworked with Excel
It cannot contribute to quality and performance improvement by securing not only work efficiency but also speed, accuracy, and reliability.
introduction performance
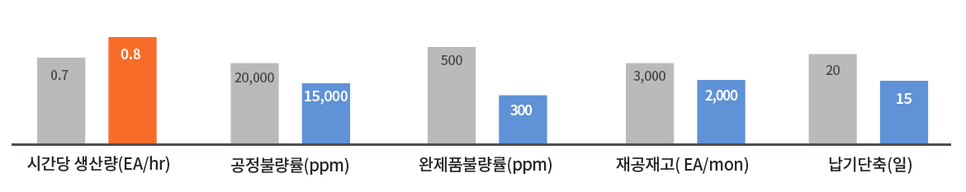
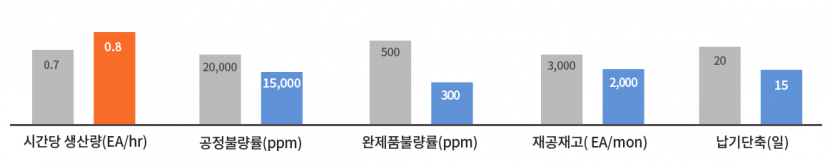
quantitative performance
qualitative performance
ㅇ Manufacturing data DB management and visibility (Stent part)
- Manufacturing information tracking and management by linking raw material warehousing information and semi-finished product warehouse warehousing information with ERP
- Manage all manufacturing conditions/start/completion/WIP information DB
ㅇ Manufacturing data DB management and visibility (Coating part)
- Complete inspection of semi-finished products according to the daily plan and manage product information tracking
- Manage all manufacturing conditions/start/completion/WIP information DB
ㅇ Manufacturing data DB management and visibility (Introducer part)
- Tracking management of loading information of Stent and Introducer, hot spot of Assy history/problem tracking after combination
ㅇ Manufacturing data DB management and visibility (Packing operation part)
- For manufacturing improvement through the combination of manufacturing history and quality inspection results before and after packing
- Tracking all information from Stent to Packing
ㅇ Improvement of production and shipment performance management
- Accurate and transparent performance management is possible by providing production performance management and shipment performance management through the barcode system
ㅇ It is expected that the real-time and accurate information sharing system will be established, which will increase productivity and increase corporate profits.
ㅇ Fast and consistent sharing of on-site data and expedited decision-making
ㅇ It is possible to monitor the flow and management activities within the production site such as production, inventory, defects, and materials in real time to ensure transparency. Better management is possible based on the acquired data
ㅇ Since the data obtained from the system is stored in a DB, it becomes a data asset that can be used as a basis for future business expansion and material purchase.
ㅇ Provide company-wide manufacturing status information to secure global medical device regulatory responsiveness through the introduction of global MES in the medical device industry, respond to design-manufacturing-quality linkages, and secure visibility between production/materials/quality/purchase infrastructure manufacturing execution operation and manufacturing/inventory Linkage Perspective Q(Quality)/C(Cost)/D(Schedule) Securing management efficiency
ㅇ Securing CSV (Computer System Validation) and responding to the Food and Drug Act due to the characteristics of medical device manufacturers that must comply with FDA and various domestic and foreign legal regulations
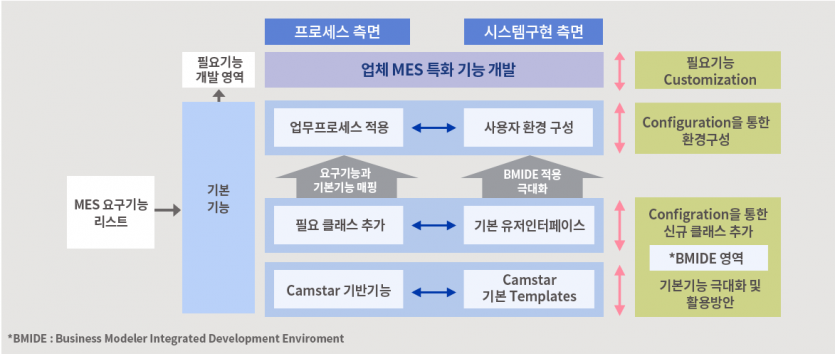
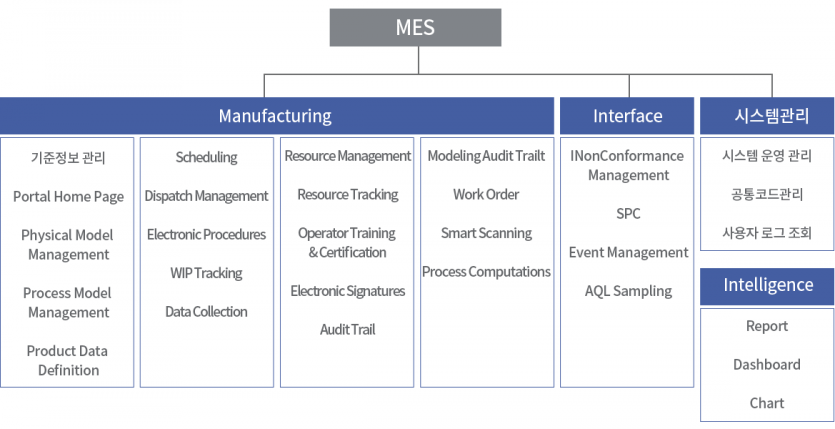
Implementation Application System






 KOR
KOR